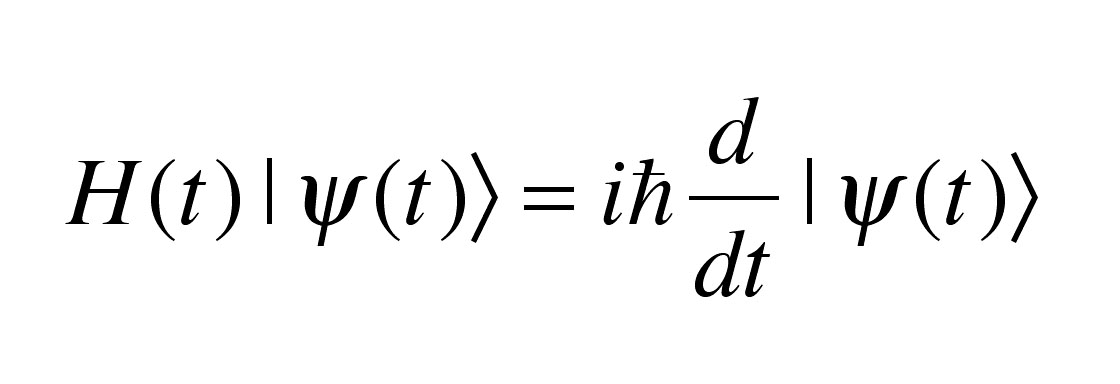
Erwin Schrödinger was one of the most influential thinkers in modern science, and his contributions to the field of quantum mechanics have shaped our understanding of the world we live in today. Today we’re taking a deep dive into Schrödinger’s life and work to uncover the brilliance that led him to become a Nobel Prize-winning physicist.
Schrödinger is best known for his “wave equation,” which he formulated in 1926 while working at the University of Vienna. It was a revolutionary idea, as it offered an alternative explanation of quantum mechanics that was seen as revolutionary at the time. From this equation emerged some of the most important advances in quantum mechanics, including Heisenberg’s uncertainty principle and Bohr’s atomic model.
However, less is known about Schrödinger’s personal life than his scientific career. Join us as we explore Erwin Schrödinger’s journey from student to Nobel laureate and unravel the life story of one incredible thinker.
The Early Life of Erwin Schrödinger
It’s impossible to learn about the famous physicist Erwin Schrödinger without first exploring his upbringing. Born and raised in Austria, he grew up in a small but well-educated family of academic parents. He was an only child and loved to study literature and music; even as a young boy, Erwin excelled academically. His hard work paid off—as a teenager, his brilliance earned him admittance to the University of Vienna, where he studied mathematics and physics.
By the time he finished his doctorate in 1910, Erwin was already considered a genius in the scientific community. His groundbreaking research focused on areas like thermodynamics and electricity theory, leading to several significant discoveries over the next few years. Most notably, he wrote an important paper on quantum theory that explored how energy is exchanged between particles—a groundbreaking topic that would later become known as wave mechanics or “Schrödinger’s equation”.
Schrödinger’s Contributions to Quantum Theory
Did you know that Erwin Schrödinger was an accomplished physicist of the 20th century? His most notable contribution was the formulation of the wave equation in 1926, which provided a foundation for quantum mechanics and wave mechanics. This equation enabled him to explain the properties of atoms and molecules in terms of waves and probabilities.
Schrödinger’s wave equation also established the principle that matter has a wave-like nature. He showed that particles at their base level are neither particles nor waves, but both at the same time—a concept known as wave-particle duality. While this notion may seem revolutionary, Schrödinger himself said that “in physics, two or three theories can exist side by side without contradiction for some time until one is finally shown to be correct and the others false”.
What made Schrödinger’s work revolutionary was that he was able to apply his theories to describe physical systems in macroscopic scale—like atoms, molecules and their interactions—in terms of their underlying principles on a quantum level. This not only gave scientists an idea of what reality is like on a subatomic level, but also helped them better understand phenomena such as radioactivity, blackbody radiation, chemical bonds and more.
Schrödinger’s Later Years and Awards
You may have heard of Erwin Schrödinger’s famous equation which explains why atoms and molecules exist in multiple states simultaneously. This pioneering discovery earned him the Nobel Prize in 1933 and was his lasting legacy, but it was only part of his outstanding career.
In the later years of his life, Schrödinger promoted the idea of “unified science” – a concept based on interdisciplinary studies that merged the sciences and humanities into one universal whole.
Schrödinger also worked on topics such as color theory, statistical mechanics, and thermodynamics, making significant contributions to these fields. In 1945 he became a professor at Oxford University, and even held positions at other prestigious universities like the University of Graz and University College Ireland.
Awards for His Work
Throughout his career, Schrödinger received many prestigious awards commending him for his brilliant work:
- Nobel Prize in Physics (1933)
- Max Planck Medal (1937)
- Austrian Decoration for Science & Art (1942)
- Faraday Lectureship (1945)
Schrödinger’s Influence on Physics and Beyond
At this point in his career, Schrödinger had already had a significant impact on atomic structure, quantum mechanics, and statistical mechanics. However, Schrödinger would continue to contribute immensely to the world of quantum theory.
In 1926, he published an article that played off of Max Born’s work and demonstrated the concept of wave mechanics. His equation suggested that particles behave like waves—something previously unheard of in the world of physics. This was the beginning of “wave mechanics” and it provided the basis for discovering new ways to understand and work with atomic particles.
This equation was revolutionary in its implications, as it offered a fresh way of looking at how particles interact with each other—not just in physics, but in all scientific fields. The effects of Schrödinger’s work can be felt across disciplines such as chemistry, electronics, materials science and beyond.
On top of this, Schrödinger also introduced new ideas to bridge classical physics and quantum theory to create a more complete view of reality that is still used by modern physicists today. In addition to this major contribution, he also wrote extensively about entropy, relativity theory and thermodynamics which led to the development of modern-day statistical methods for chemists and biologists.
His Impact on Modern Quantum Mechanics
Erwin Schrödinger’s contributions to Quantum Mechanics have been invaluable. His work on wave mechanics and his famous equation are proof of his profound understanding of the world of subatomic particles. His efforts to find answers to some of the most difficult questions in physics greatly impacted modern Quantum Mechanics.
Wave Mechanics
Schrödinger developed a comprehensive mathematical framework that described the behavior of quantum systems, known as Wave Mechanics. He explored the idea that electrons could be described as waves instead of particles, paving the way for a better understanding of Quantum Mechanics and quantum-scale phenomenon.
Schrödinger Equation
In 1926, Schrödinger introduced an equation which provided a theoretical way to describe atomic behavior. This iconic equation describes how particles with wave-like properties, such as electrons, can interact with each other and with other particles in a quantum system. The equation has become one of the cornerstones of modern Physics and is widely used by scientists today.
Schrödinger’s immense contributions to Modern Physics are undeniable—his work revolutionized our understanding of atomic structure and behavior, leading us closer to unlocking the secrets of our universe.
Celebrating the Life of Erwin Schrödinger
You have been learning about Erwin Schrödinger, the Austrian physicist who made a lasting impact on science. Throughout his career, he made many discoveries and contributions to quantum mechanics, thermodynamics and quantum field theory that are still relevant today. In order to fully appreciate the life and work of this brilliant scientist, it is important to learn more about his accomplishments.
Nobel Prize Achievements
Erwin Schrödinger was awarded the 1933 Nobel Prize in Physics for discovering the wave mechanics formulation of the Schrödinger equation (often referred to as the Schrödinger Wave Equation). This was a major breakthrough in understanding how atomic particles behave at their most basic level. He also earned a second Nobel Prize in 1963 for his research on thermodynamics.
Scientific Contributions
Schrödinger’s contributions to science were vast, but one of his most important contributions was developing an equation — now known as the Schrödinger’s equation — which provided a mathematical description of how atoms behave. This groundbreaking work laid the foundation for much of modern physics and is still used and studied today. He also developed several other theories related to atoms including wave equations, as well as theories relating to quantum field theory and thermodynamics.
Legacy
Erwin Schrödinger’s groundbreaking work helped pave the way for further discoveries in quantum physics, which have had a profound impact on our understanding of particle physics and the structure of matter at its most basic level. His legacy lives on through his works and through those who continue to build upon them even today.
In short, Erwin Schrödinger was one of the most important and influential physicists of the twentieth century. His profound insights into quantum theory revolutionized the field of physics and laid the groundwork for modern advancements in the field. Although he faced many personal and professional challenges throughout his life, his contributions to science remain unparalleled and continue to shape our understanding of the universe today. Schrödinger’s legacy proves that a single person can have a profound impact on the world and will remain an inspiration for centuries to come.
